Mission
The University of Puerto Rico (UPR) and CDI Laboratories have partnered to provide comprehensive solutions for process development. The CDI facility, based in Mayagüez, performs protein expression trials and develops purification schemes. The UPR facility, based in the Molecular Sciences Research Center, develops tailored analytical tools for a wide array of biological products in a good manufacturing practice environment. Our goal is to streamline the process development pipeline of a promising biotherapeutic from lead to clinic.
Protein purity is monitored using the microchip capillary gel electrophoresis technology.


The gel-style output for the bovine serum albumin (BSA) run in triplicate
CHO host cell proteins are quantified using sensors prepared with CHO specific antibodies and biolayer interferometry.

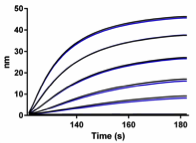
The standard curve (0 to 200 ng/ml) run in duplicate
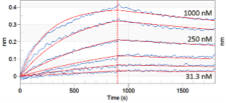
We determine lot-to-lot variability of protein functionality by monitoring binding affinity using the biolayer interferometry technology.
Data and curve-fits for an antibody-antigen pair
Sugars create heterogeneity in biopharmaceuticals. We can monitor this heterogeneity by charge analysis using an imaged capillary isoelectric focusing system.
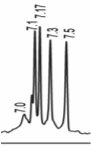

Isoelectric variants of hemoglobin
Protein expression optimization is sequentially performed in shaker flasks, parallel mini-reactors and standard bioreactors (up to 10L) for rapid transition to large-scale production. Protein purification optimization is carried out utilizing an AKTA system to ensure comprehensive data acquisition and reproducibility.

We employ a MALDI MS ToF/ToF to determine protein whole mass and to identify proteins by peptide mass fingerprinting.

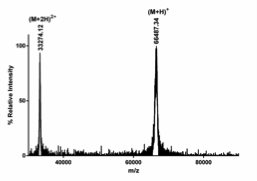
BSA whole mass determination
Glycans can confound the development of biological products due to their heterogeneity. We monitor released glycans by MALDI mass spectrometry.
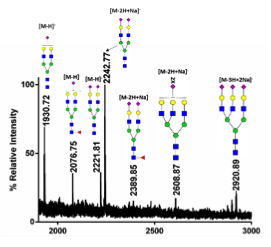 The acidic glycans from BaL virions (Sample courtesy of Dr. Loyda Melendez)
The acidic glycans from BaL virions (Sample courtesy of Dr. Loyda Melendez)